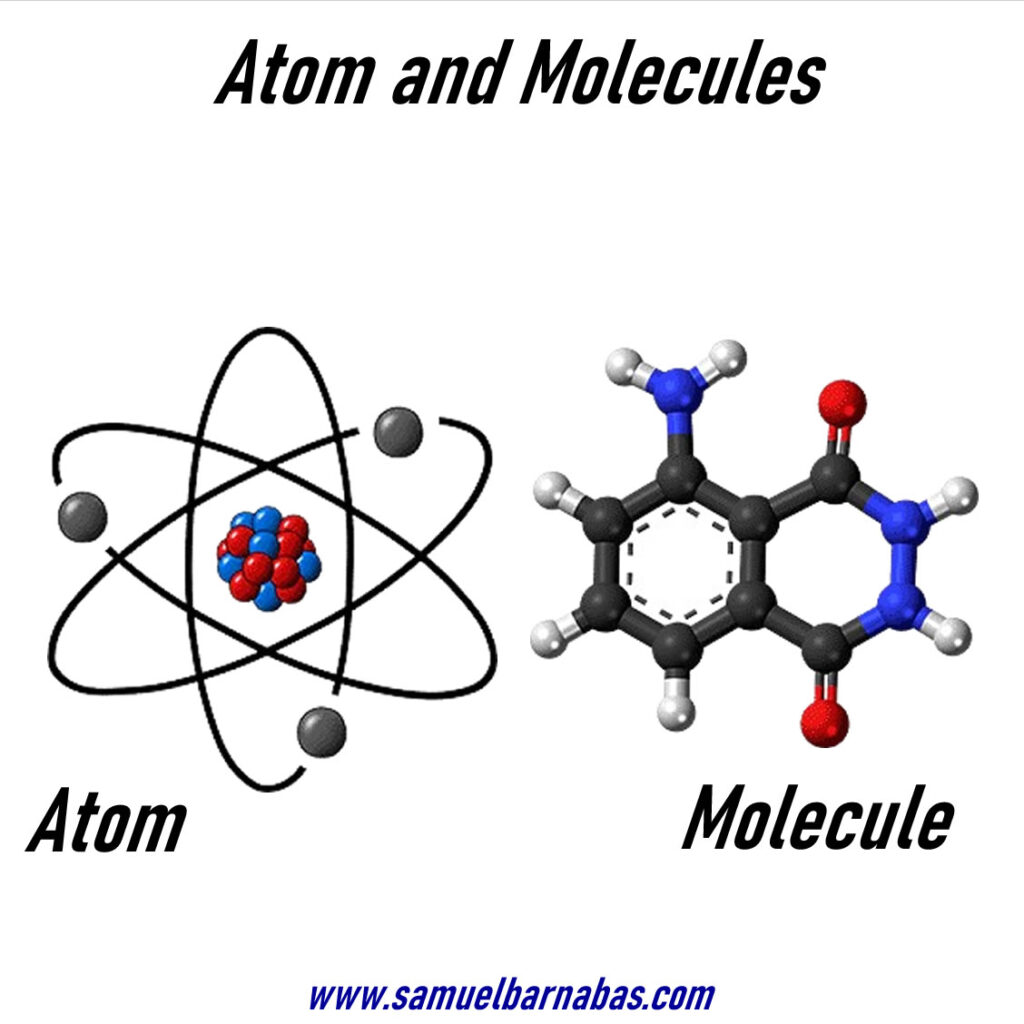
Atoms and Molecules
What is an Atom? (Atom Definition)
Atoms are defined as “the basic building blocks of matter”.
Atoms are the fundamental units of all matter. They are exceedingly small and comprise even tinier particles known as neutrons, protons, and electrons. These particles combine to form atoms, which then join together with other atoms to create matter. It requires numerous atoms to construct anything tangible.
An atom is the smallest indivisible unit of matter possessing the characteristic properties of a chemical element. However, atoms do not exist independently; rather, they form ions and molecules. These ions and molecules then combine in vast numbers to create the matter that we perceive, interact with, and touch.
What is a Molecule? (Molecule Definition)
A group of two or more atoms is linked together by sharing electrons in a chemical bond.
A molecule is formed when two or more atoms are strongly bound together by attractive forces or chemical bonds. The term “atom” signifies the smallest unit of matter that can exist independently. “Valency” denotes an element’s ability to combine with other elements.
|
Atoms and Molecules Definition
Atoms are far too minuscule to be visible; therefore, experiments are conducted with large quantities of them to determine their structure and behavior. Based on the outcomes of these experiments, we can create a theoretical model of an atom that mimics its actual behavior.
Molecules are formed by one or more atoms joined together by covalent (chemical) bonds. Atoms can be represented by circular shapes, each containing a nucleus at its center (comprising protons and neutrons). Surrounding the nucleus are one or more concentric circles denoting the ‘shells’ or ‘levels’ where electrons reside, with markings indicating the electrons at each level. A molecule is the smallest unit of a substance that maintains its identity, consisting of two or more atoms bonded together chemically.
Atoms and Molecules
Atom Definition Chemistry
The smallest particle of an element, which may or may not have an independent existence but always takes place in a chemical reaction is called an atom.
An atom is the smallest unit of an element that retains its characteristic properties. It is composed of subatomic particles that cannot be created or destroyed. All atoms of the same element are identical, while different elements have distinct types of atoms. Chemical reactions occur when atoms rearrange.
Atoms consist of three fundamental particles: protons, neutrons, and electrons. Neutrons and protons have similar masses, whereas the mass of an electron is negligible. Protons carry a positive charge, neutrons are neutral, and electrons have a negative charge. An atom contains an equal number of protons and electrons, resulting in a neutral overall charge. The nucleus, located at the center of the atom, contains protons and neutrons, giving the atom its mass and positive charge. Electrons occupy the space around the nucleus, with most of the mass concentrated within the nucleus.
The nucleus, the center of the atom, houses neutrons and protons, which contribute to the atom’s weight and positive charge. Neutrons carry no charge and have a mass of one unit, while protons carry a single positive charge and also have a mass of one unit. The atomic number of an element corresponds to the number of protons, or positive charges, in its nucleus. The atomic weight is determined by the total number of protons and neutrons in the nucleus. Electrons, carrying a single negative charge, orbit the nucleus in layers resembling the shells of an onion.
What is the Size of an Atom?
The dimensions of an atom are incredibly minute, far smaller than what we can envision. If millions of atoms are stacked together, they would create a layer as thin as a sheet of paper. Measuring the size of an isolated atom is challenging due to the elusive nature of electrons orbiting the nucleus.
Nevertheless, we can estimate the size of an atom by assuming that the distance between neighboring atoms is approximately half the atomic radius. Atomic radius is typically quantified in nanometers.
Relative Sizes
| Examples | Radii (m) |
| Atom of Hydrogen | 10−10 |
| Molecule of water | 10−9 |
| Molecule of hemoglobin | 10−8 |
| Grain of Sand | 10−7 |
What are Atoms made of?
An atom consists of three fundamental particles: neutrons, protons, and electrons. It’s worth noting that hydrogen is an exception, as it lacks neutrons.
- Every atom possesses a nucleus surrounded by one or more electrons.
- The nucleus generally contains an equal number of protons and neutrons, collectively called nucleons.
- Protons bear a positive charge, electrons carry a negative charge, and neutrons have no charge, remaining neutral.
What is Atomic Mass?
The atomic mass refers to the mass of an atom within a specific chemical element. It is approximately equal to the combined total of neutrons and protons present in the atom. This measurement is typically expressed in atomic mass units (denoted as u). One atomic mass unit (amu) is precisely one-twelfth of the mass of a single atom of carbon-12. The relative atomic masses of elements are determined in relation to the carbon-12 atom.
Atomic masses of Some Elements
| Elements | Atomic Mass (u) |
| Hydrogen | 1 |
| Carbon | 12 |
| Nitrogen | 14 |
| Oxygen | 16 |
| Sodium | 23 |
| Magnesium | 24 |
| Sulfur | 32 |
| Chlorine | 35.5 |
| Calcium | 40 |
Salient features of Dalton’s Atomic Theory
- – Matter is made up of tiny particles called atoms.
– Atoms are indivisible entities that cannot be created or destroyed in chemical reactions.
– All atoms of the same element possess identical chemical properties and mass.
– Atoms of different elements exhibit varied chemical properties and masses.
– Atoms combine in ratios of small whole numbers to create compounds.
Matter encompasses everything in our surroundings and is comprised of basic structural units. To illustrate this concept, consider a storybook as an example. The book consists of multiple pages, each page containing paragraphs, and each paragraph comprising sentences.
Further breaking it down, each sentence contains numerous words, and each word consists of characters. Similarly, matter is composed of substances that contain molecules. These molecules, in turn, are composed of groups of atoms.
Atoms, in simple terms, are described as the smallest units of matter. In ancient times, scientists pondered the divisibility of matter. Around 500 BC, the concept of matter’s divisibility emerged in India. A scientist named Maharishi Kanad proposed that matter could be divided into smaller and smaller units. The smallest unit of matter beyond which further division was deemed impossible was known as “parmanu.”
What is a Molecule?
Molecule Definition
A molecule is defined as the smallest unit of a compound that contains the chemical properties of the compound.
Molecules are composed of assemblies of atoms. Explaining the structure of an atom, it is further divided into smaller units. Protons, electrons, and neutrons constitute the sub-particles of an atom. Protons and neutrons reside within the nucleus of the atom while electrons orbit around the nucleus.
Protons are particles with a positive charge, whereas electrons are negatively charged particles. Neutrons carry no charge, rendering the nucleus positively charged due to the presence of protons. The nucleus, situated at the center of an atom, constitutes the bulk mass. Atoms are predominantly empty space.
Every element possesses a distinct atomic number, which signifies the number of protons present in its nucleus, denoted by Z.
Regarding the mass of atoms, the combined mass of their constituent particles is considered. Electrons have negligible mass. Therefore, the mass of an atom is the summation of the masses of protons and neutrons, denoted by A.
A molecule stands as the smallest unit or particle of a compound that exhibits the physical and chemical properties of that compound. This does not imply that molecules cannot be disassembled into smaller components, such as the constituent atoms or fragments of the molecule, each comprising multiple atoms or parts of atoms.
In summary, molecules are assemblies of atoms, and atoms are further divided into smaller units consisting of protons, electrons, and neutrons. Protons and neutrons reside in the nucleus, while electrons orbit around it.
Examples of Molecules
A molecule is an assembly of two or more atoms, forming the smallest identifiable unit into which a pure substance can be divided while retaining its composition and chemical properties. Here are a few examples of molecules:
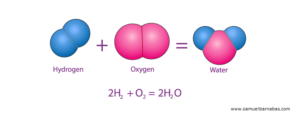
- H2O (water)
- N2 (nitrogen)
- O3 (ozone)
- CaO (calcium oxide)
- C6H12O6 (glucose, a type of sugar)
- NaCl (table salt)
Forces between Atoms and Molecules
The most basic forces between atoms stem from electron transfer. An example is sodium fluoride (NaF). The sodium (Na) atom possesses a nuclear charge of +11, with 2 electrons in the K shell, 8 in the L shell, and 1 in the M shell. Meanwhile, the fluorine (F) atom holds a nuclear charge of 9, with 2 electrons in the K shell and 7 in the L shell.
In this scenario, the outermost electron of the sodium atom readily transfers to the fluorine atom. As a result, both atoms achieve a complete shell, but the sodium atom now carries a net charge of +1, while the fluorine atom bears a net charge of -1. These ions subsequently attract each other due to direct Coulombic interaction. The force between them is potent, varying as x^-2, where x represents the distance separating the ions, and it acts along the line connecting the ions.
Frequently Asked Questions – FAQs
How do atoms become molecules?
When atoms combine to form molecules, they are held together by chemical bonds. These bonds are formed by the sharing or exchanging of electrons between the atoms. It is important to note that only the electrons in the outermost shell of the atoms are involved in bonding.
What is a simple molecule?
Water is a fundamental molecule composed of a few atoms. Basic molecular substances are compounds where strong covalent bonds connect the atoms. However, weak forces also hold these molecules together, resulting in high melting and boiling points.
Is ozone a molecule?
Ozone is a molecule consisting of three oxygen atoms. Its chemical symbol is O3, as the symbol for the oxygen atom is O. The majority of the ozone found in our atmosphere is produced by the interaction of oxygen molecules with ultraviolet radiation emitted by the sun.
Can a molecule have one atom?
A molecule is defined as an electrically neutral group of two or more atoms held together by chemical bonds. Therefore, by its very definition, a molecule cannot be formed from a single atom.
What is the structure of an atom?
Atoms consist of three fundamental particles: protons, electrons, and neutrons. The nucleus of the atom, located at its center, contains the protons, which are positively charged, and the neutrons, which are neutral. Surrounding the nucleus are electron shells, which contain the negatively charged electrons.
Is Salt a molecule?
Molecules are held together by molecular bonds. For instance, table salt (NaCl) is considered a compound because it is composed of more than one type of element (sodium and chlorine). However, it is not a molecule because it is held together by an ionic bond. We can describe sodium chloride as an ionic compound.
What is Atom and example?
Many atoms are composed of a positively charged nucleus containing protons and neutrons, surrounded by a cloud of negatively charged electrons. An atom is the fundamental unit of matter, consisting of at least one proton. Examples of atoms include hydrogen (H) and neon (Ne).
What is the work of an atom?
The protons and neutrons are densely packed in the central region of the atom, known as the nucleus, while the much smaller electrons orbit around the nucleus. Visual representations of atoms often depict electrons resembling satellites orbiting around a planet, akin to the Earth and its satellites.
What is the difference between atoms and molecules?
An atom is the minute building block of a chemical element, existing either independently or as part of a larger structure. Molecules, on the other hand, are groups of atoms held together by chemical bonds, representing the smallest unit of a compound. These molecules can consist of two or more identical or different atoms that are chemically bonded together.