Chemical Reaction
What is a Chemical Reaction?
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance.
Chemical reactions permeate our surroundings, from the intricate metabolic processes that break down food in our bodies to the solar radiation generated by chemical reactions in the sun. Prior to delving into chemical reactions, understanding both physical and chemical changes is essential.

The burning candle serves as an exemplary illustration of both physical and chemical changes. When a candle is lit, the process of burning represents a chemical change, resulting in the transformation of wax into other substances such as carbon dioxide and water vapor. On the other hand, the conversion of the candle into wax as it melts represents a physical change, where the state of the substance alters without any change in its chemical composition. Notably, in a physical change, the substance typically undergoes a change in state, whereas in a chemical change, new substances are often formed, accompanied by the release or absorption of energy. It’s worth noting that chemical changes are often associated with certain physical alterations, highlighting the interconnectedness of these phenomena.
Basic Concepts of Chemical Reactions
- A Chemical Reaction is a process that occurs when two or more molecules interact to form a new product(s).
- Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
- Chemical reactions play an integral role in different industries, customs and even in our daily life. They are continuously happening in our general surroundings; for example, rusting of iron, pottery, fermentation of wine and so on.
- In a chemical reaction, a chemical change must occur which is generally observed with physical changes like precipitation, heat production, colour change etc.
- A reaction can take place between two atoms or ions or molecules, and they form a new bond and no atom is destroyed or created but a new product is formed from reactants.
- The rate of reaction depends on and is affected by factors like pressure, temperature, the concentration of reactants.
Chemical Equations
Due to the vast amounts of chemical reactions happening around us, a nomenclature was developed to simplify how we express a chemical reaction in the form of a chemical equation. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants while stating certain condition for which how the reaction has been conducted.
The reactants are on the left-hand side whereas the products formed are on the right-hand side. The reactants and products are connected by a one-headed or two-headed arrows. For example, a reaction
A + B → C + D
Here, A and B are the reactants, which react to form the products C and D. In an actual chemical equation, reactants are denoted by their chemical formula. In order to assure the law of conservation of mass, a chemical equation must be balanced i.e. the number of atoms on both sides must be equal. This is the balancing of the equation.
Let us consider an actual chemical reaction between Methane(CH₄) and Oxygen (O2),
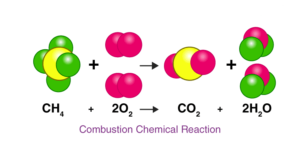
Here we can see how the number of each atom on the left side is balanced on the right side, as stated by the law of conservation of mass.
Types of Chemical Reactions
The basis for different types of reactions is the product formed, the changes that occur, the reactants involved and so on. Different types of reactions are
- Combustion reaction
- Decomposition reaction
- Neutralization reaction
- Redox Reaction
- Precipitation or Double-Displacement Reaction
- Synthesis reaction
1. Combustion Reaction
A combustion reaction involves the reaction of a combustible material with an oxidizer, typically oxygen, to produce an oxidized product. One example is the combustion of magnesium metal.
In this reaction, two magnesium atoms react with a molecule of oxygen to produce two molecules of the compound magnesium oxide. This process releases heat as an exothermic reaction. The balanced chemical equation for this combustion reaction is:
2Mg(s) + O2(g) → 2MgO(s)
Here, magnesium (Mg) reacts with oxygen (O2) gas to form magnesium oxide (MgO) as a solid product. This reaction is highly exothermic, meaning it releases heat energy as a byproduct of the chemical reaction.
2. Decomposition Reaction
A decomposition reaction is a chemical reaction where a single compound breaks down into multiple products. This process often requires an input of energy, such as heat, light, or electricity, to break the bonds within the compound. One example is the decomposition of calcium carbonate, which yields calcium oxide (quicklime), a major component of cement.
The balanced chemical equation for this decomposition reaction is:
CaCO3(s) → CaO(s) + CO2(g)
Here, calcium carbonate (CaCO3) decomposes into calcium oxide (CaO), also known as quicklime, and carbon dioxide (CO2). This reaction typically requires heating to initiate the decomposition process, and it is commonly used in the production of lime and cement.
Here, the compound Calcium carbonate when heated breaks down into Calcium Oxide and Carbon Dioxide.
3. Neutralization Reaction
A neutralization reaction is indeed a chemical reaction between an acid and a base, resulting in the formation of a salt and water as products. The water molecule formed originates from the combination of hydroxide ions (OH⁻) from the base and hydrogen ions (H⁺) from the acid. In such reactions, the overall pH of the resulting solution is approximately 7, indicating neutrality.
Consider the example of the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH), which yields sodium chloride (NaCl), commonly known as table salt, and water as products. The balanced chemical equation for this reaction is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Here, hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to produce sodium chloride (NaCl) and water (H2O). This reaction demonstrates the fundamental principles of neutralization reactions, where the acidic and basic properties of the reactants are neutralized, resulting in a salt and water.
4. Redox Reaction
A reduction-oxidation (redox) reaction is indeed a chemical reaction where there is a transfer of electrons between chemical species. Consider the example of an electrochemical cell involving a redox reaction between zinc and hydrogen.
The balanced chemical equation for this redox reaction is:
Zn(s) + 2H⁺(aq) → Zn²⁺(aq) + H₂(g)
Here, a zinc atom (Zn) reacts with two positively charged hydrogen ions (H⁺) in an aqueous solution. During the reaction, electrons are transferred from the zinc atom to the hydrogen ions, resulting in the formation of zinc ions (Zn²⁺) and hydrogen gas (H₂). This transfer of electrons from zinc to hydrogen ions represents the reduction of hydrogen ions (gain of electrons) and the oxidation of zinc (loss of electrons), hence it’s a redox reaction.
Redox reactions involve the simultaneous occurrence of reduction (gain of electrons) and oxidation (loss of electrons) processes, and they are crucial in various chemical and electrochemical processes.
5. Precipitation or Double-Displacement Reaction
Indeed, the reaction between silver nitrate and sodium chloride is a classic example of a double displacement reaction, also known as a metathesis reaction. In this type of reaction, the anions and cations of two compounds exchange places to form two new compounds. The balanced chemical equation for this reaction is:
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
Here, silver nitrate (AgNO3) reacts with sodium chloride (NaCl) in an aqueous solution. The silver ion (Ag⁺) from silver nitrate combines with the chloride ion (Cl⁻) from sodium chloride to form silver chloride (AgCl), which precipitates as a solid. Meanwhile, the sodium ion (Na⁺) from sodium chloride combines with the nitrate ion (NO3⁻) from silver nitrate to form sodium nitrate (NaNO3) in the solution.
This double displacement reaction results in the formation of two new compounds: silver chloride (AgCl) and sodium nitrate (NaNO3). Double displacement reactions are common in chemistry and are used in various applications, including precipitation reactions and the synthesis of new compounds.
Here, Silver Nitrate and Sodium Chloride undergo a double displacement reaction. Wherein Silver replaces Sodium in Sodium Chloride and Sodium joins with Nitrate becoming Sodium Nitrate along with the Silver Chloride as the product.
6. Synthesis Reaction
A synthesis reaction, often referred to as a combination reaction, is a fundamental type of chemical reaction where multiple simple compounds combine under specific physical conditions to form a more complex product. The resulting product is always a compound.
Consider the synthesis reaction of sodium chloride, where solid sodium reacts with chlorine gas:
2Na(s) + Cl2(g) → 2NaCl(s)
In this reaction, two atoms of solid sodium (Na) combine with one molecule of chlorine gas (Cl2) to produce two molecules of sodium chloride (NaCl), commonly known as table salt. This synthesis reaction demonstrates the basic principle of combination reactions, where simpler substances come together to form a more complex compound.
Important Points to Remember
- In a chemical change, a new compound is formed but in a physical change, the substance changes its state of existence.
- Atoms or ions or molecules which react to form a new substance are called reactants; the new atoms or molecules formed are products.
- A chemical reaction follows the law of conservation of mass. That is no atom is destroyed or created but only a new product is formed from reactants.

