Aldehydes, Ketones and Carboxylic Acids
What are Aldehydes, Ketones, and Carboxylic Acids?
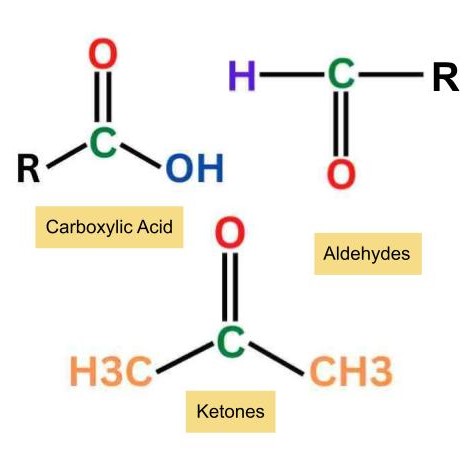
Aldehydes, ketones, and carboxylic acids are classified as carbonyl compounds due to their inclusion of a carbon-oxygen double bond. These organic compounds hold significant importance in organic chemistry and find widespread industrial applications.
The shared presence of the carbonyl group in both classes of compounds results in similar chemical properties. However, aldehydes exhibit higher reactivity compared to ketones owing to the presence of a free hydrogen atom.
Compounds featuring a carbon-oxygen double bond, known as the carboxyl group, constitute one of the most crucial functional groups in organic chemistry. The carbonyl group stands out as a vital component in compounds within living systems.
What are Aldehydes?
Aldehydes are organic compounds which have the functional group -CHO.
Carbonyl compounds are composed of a central carbonyl carbon that is doubly bonded to an oxygen atom and singly bonded to an R group (which can be any alkyl group) as well as a hydrogen atom.
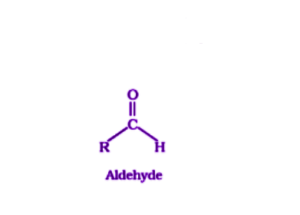
Where R stands for alkyl or aryl group.
Preparation of Aldehydes
Acid chlorides can be reduced to aldehydes using hydrogen in the presence of a palladium catalyst dispersed on barium sulfate.
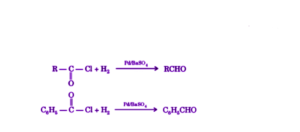
The described reaction is commonly referred to as the Rosenmund reduction, primarily employed for the synthesis of aromatic aldehydes. It is noteworthy that this reaction cannot be utilized for the production of ketones and formaldehyde.
Properties of Aldehydes
- The structure of aldehydes reveals a central carbon atom with sp2 hybridization, forming a double bond with oxygen and a single bond with hydrogen.
- Small aldehydes, such as formaldehyde and acetaldehyde, demonstrate considerable solubility in water, making them industrially significant.
- Aldehydes commonly display a propensity for oligomerization or polymerization.
- Due to the electron-withdrawing nature of the carbonyl center, the aldehyde group exhibits some polarity.
Nomenclature of Aldehydes
Acyclic and aliphatic aldehydes are named based on their longest carbon chain with the suffix “-al”. For instance, CH3CH3CH2CHO is named butanal due to its four-carbon chain.
When the aldehyde functional group is incorporated into a ring, the suffix “-carbaldehyde” must be utilized. For example, C6H11CHO is named cyclohexanecarbaldehyde.
In the case of natural compounds or carboxylic acids, the prefix “-oxo” is employed to denote the carbon that constitutes the aldehyde functional group. For instance, (CHO)-CH2COOH is named 3-oxopropanoic acid.
Some common and IUPAC names for some aldehydes are tabulated below.
| Formula | Common name | IUPAC name |
| HCHO | Formaldehyde | Methanal |
| CH3CHO | Acetaldehyde | Ethanal |
| CH3-CH(CH3)-CHO | Isobutyraldehyde | 2-Methylpropanal |
| CH3-CH=CH-CHO | Crotonaldehyde | 2-Butenal |
Uses of Aldehydes
- Formaldehyde is used as a disinfectant and as a preservative for biological specimens.
- Aldehyde is used for silvering mirrors.
- Formaldehyde is used for the production of a variety of plastic and resins.
- Benzaldehyde is used in perfumery and in the dye industry.
What are Ketones?
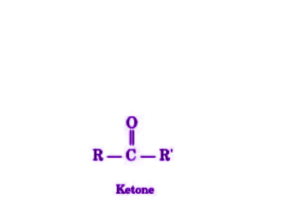
Ketones are organic compounds which have the functional group C=O and the structure R-(C=O)-R’.
Ketones are carbonyl compounds characterized by carbon-containing substituents on both sides of the carbon-oxygen double bond. The carbonyl carbon within the ketone group exhibits sp2 hybridization. The structure of ketones is trigonal planar, with the carbonyl carbon at the center. The bond angles of this structure approximate 120 degrees. Due to the polar nature of the carbon-oxygen bond (with oxygen being more electron-withdrawing than carbon), ketones tend to exhibit nucleophilic behavior at the oxygen atom and electrophilic behavior at the carbon atom.
Ketones are widely manufactured on an industrial scale for their utility as solvents, pharmaceuticals, and as raw materials for polymer synthesis. Notable ketones include methyl ethyl ketone, also known as butanone, cyclohexanone, and acetone.
Preparation of Ketones
Acid chlorides on reaction with dialkyl cadmium produce ketones. Dialkyl cadmium themselves are prepared from Grignard reagents.
2R-Mg-X + CdCl2 → R2Cd + 2 Mg(X)Cl
2RCOCl + R2Cd → 2R-CO-R + CdCl2
The method is useful in a way that the mixed ketones can be prepared very conveniently.
Properties of Ketones
- Ketones are polar in nature due to the presence of a polar carbonyl group. Therefore they have higher boiling points than non-polar compounds.
- It cannot form any intermolecular hydrogen bond-like alcohols because there is no hydrogen attached to an oxygen atom.
- Ketones have large dipole moments compared to alcohols or ethers due to the shifting of pi electrons.
- Ketones react with hydrogen cyanide to form cyanohydrins. The reaction is normally carried out in the presence of a base, which acts as a catalyst in the absence of a base the reaction proceeds slowly.
- Most of the ketones form bisulphite addition products when it is added to sodium bisulphite.
Nomenclature of Ketones
- Ketones are named after their parent alkanes with the suffix “-anone”. The carbonyl group’s position in the ketone is denoted by a number while naming the ketone. For example, CH3(CO)CH3 is called 2 propanone. However, this compound is generally referred to as acetone.
- Commonly, ketones are named by writing the name of each individual alkyl group attached to the carbonyl carbon and then “ketone” as the third word of the name. For example, butanone can be written as methyl ethyl ketone.
| Formula | Common name | IUPAC name |
| CH3-C(O)-CH3 | Acetone | Propanone |
| CH3-C(O)-CH2-CH3 | Ethylmethylketone | Butanone |
| CH3-CH2-C(O)-CH2-CH3 | Diethyl ketone | Pentan-3-one |
| CH3-CH(CH3)-C(O)-CH3 | Isopropyl methyl ketone | 3-Methylbutan-2-one |
Uses of Ketones
- Propanone is used to make polymers for example perspex.
- ketones are used as solvents and as a starting material for the synthesis of many organic compounds.
- Acetone and ethyl methyl ketone is mainly used as industrial solvents.
What is Carboxylic Acid?
Carboxylic acids are organic compounds that contain a (C=O)OH group attached to an R group (where R refers to the remaining part of the molecule).
The COOH group is commonly referred to as a carboxyl group. Carboxylic acids can generally be expressed via the formula R-COOH. Carboxylic acids have a polar nature. They can also participate in hydrogen bonding owing to their hydrogen bond accepting nature of the C=O group and the hydrogen bond donating nature of the O-H bond. They generally have higher boiling points when compared to water and tend to form stable dimers.
Carboxylic acids play an important role in the production of pharmaceuticals, food additives, solvents, and polymers. Acetic acid, adipic acid, and citric acid are a few carboxylic acids which are extremely useful industrially.
Preparation of Carboxylic Acids
Primary alcohols are readily oxidized to carboxylic acids with common oxidizing agents such as potassium permanganate in neutral acidic or alkaline media or by potassium dichromate and chromium trioxide in acidic media.
RCH2OH → RCOOH
CH3(CH2)8CH2OH → CH3(CH2)8COOH
Properties of Carboxylic Acids
- Carboxylic acids are polar compounds and can extensively enter into hydrogen bonding.
- Aromatic carboxylic acids are practically insoluble in cold water. All carboxylic acids are soluble in organic solvents like ether, alcohol, benzene, etc.
- Among organic acids, carboxylic acids are the most acidic, but they are less acidic than the mineral acids, namely nitric acid and sulphuric acid.
Nomenclature of Carboxylic Acids
- Carboxylic acids are named by adding an “-oic acid” suffix to their parent chain. For example, C3H7COOH is called butanoic acid.
- Even if there are other substituents present, the carboxylic acid can be considered at the first position of the parent chain, as seen in the name for 3-Chloropropanoic acid.
- The COOH group can also be called “carboxy” and used as a substituent in the name of the parent structure. For example, 2-Furoic acid is commonly referred to as 2-carboxy furan.
| Formula | Common name | IUPAC name |
| HCOOH | Formic acid | Ethanoic acid |
| CH3COOH | Acetic acid | Ethanoic acid |
| CH3CH2COOH | Propionic acid | Propanoic acid |
| C6H5COOH | Benzoic acid | Benzenecarboxylic acid |
Frequently Asked Questions – FAQs
Do carboxylic acids react with aldehydes?
The carbonyl groups present in aldehydes and ketones can undergo oxidation to produce the next “oxidation level” compound, which is the carboxylic acid. When water is added to an aldehyde or ketone, a product known as a hydrate or gemdiol, characterized by the presence of two OH groups on one carbon, is formed. This reaction is catalyzed by both acids and bases.
Are ketones and aldehydes carboxylic acid derivatives?
The distinguishing feature between carboxylic acid derivatives and aldehydes/ketones lies in the presence of a functional group containing a negatively charged heteroatom (typically oxygen, nitrogen, or sulfur) directly linked to the carbonyl carbon atom in carboxylic acid derivatives. Conceptually, carboxylic acid derivatives can be envisioned as being bilateral due to this structural arrangement.
Which is more acidic aldehyde or ketone?
Compared to the alkyl groups of ketones, aldehydes exhibit greater acidity (lower pKa values) owing to the diminished electron-donating effect of protons.
What does Schiff’s test for?
The Schiff test is a chemical assay utilized to detect the presence of aldehydes within a given analyte. It involves mixing the analyte with a small quantity of Schiff reagent, facilitating the identification of aldehydes based on specific chemical reactions.
How do you purify aldehydes?
The solid aldehyde can be dissolved in ether and purified using the described method. Alternatively, they can undergo steam distillation, followed by sublimation and crystallization from toluene or petroleum ether for purification.
In conclusion, aldehydes, ketones, and carboxylic acids hold significant importance as carbonyl compounds, both industrially and in laboratory settings. Their nomenclature can be conducted according to IUPAC guidelines or by adhering to common practices.