Acid, Base and Salts
What are Acids, Bases, and Salts?
Many acids and bases occur naturally in nature, such as citric acid in fruits like orange, lemon, etc, tartaric acid in tamarind, malic acid in apples, and lactic acid in milk and milk products, hydrochloric acid in gastric juices.
Similarly, various bases are present, such as lime water. Many of these acids are commonly utilized in our daily lives, for instance, vinegar or acetic acid in culinary applications, boric acid in laundry care, baking soda for cooking purposes, and washing soda for cleaning tasks.
Moreover, several acids and bases are employed in laboratories and industries, such as hydrochloric acid (HCl), sulfuric acid (H2SO4), sodium hydroxide (NaOH), and potassium hydroxide (KOH). When these acids and bases are combined in appropriate proportions, a neutralization reaction occurs, resulting in the formation of salt and water. Some naturally occurring salts found in nature include sodium chloride (NaCl) and potassium chloride (KCl) in seawater and natural rock formations. This section will delve deeper into the properties of acids, bases, and salts.
Definitions
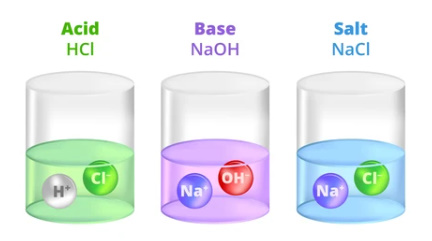
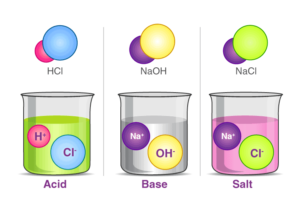
- Acid:- An acid is characterized as a substance whose aqueous solution has a sour taste, turns blue litmus paper red, and has the ability to neutralize bases.
- Base:- A base is identified as a substance whose aqueous solution tastes bitter, turns red litmus paper blue, or has the capacity to neutralize acids.
- Salt:- Salt is described as a neutral substance whose aqueous solution does not alter the color of litmus paper.
Acids
The term “acid” originates from the Latin word “acidus” or “acere,” which translates to sour. The most prominent characteristic of acids is their sour taste. An acid is a substance that releases ionizable hydronium ions (H3O+) when dissolved in water. It causes blue litmus paper to turn red. Acids dissociate in aqueous solutions to form their constituent ions, as illustrated by the following examples.
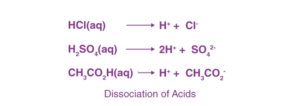

Based on their occurrence, acids are categorized into two types: natural acids and mineral acids.
Natural Acids: These acids are sourced from natural origins, such as fruits and animal products. Examples include lactic acid, citric acid, and tartaric acid.
Mineral Acids: Mineral acids are acids synthesized from minerals. Examples include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3), among others.
Check out ⇒ Dilute Acids
Bases
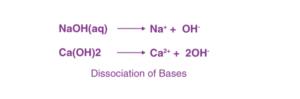
The primary characteristic of bases is their bitter taste and slippery or soapy feel. A base is defined as a substance that produces hydroxide ions (OH⁻) when dissolved in water. Bases cause red litmus paper to turn blue.

Bases dissociate in their aqueous solution to form their constituent ions, as demonstrated by the following examples.
Salts
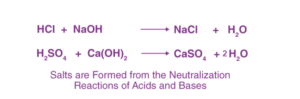
Salt is an ionic compound formed as a result of the neutralization reaction between acids and bases. Salts consist of positively charged ions, referred to as cations, and negatively charged ions, known as anions, which can be either organic or inorganic in nature. These ions are present in relative amounts, resulting in the salt having a neutral nature.

The formation of salt can be observed in the chemical reactions depicted in the equations below.