What are Carbon and its Compounds?
The molecules of a carbon compound must contain an atom of carbon. All living things, including plants and animals, are composed of organic components, which are carbon-based substances.
Carbon compounds can be found ubiquitously, from the food we consume to the fabrics we wear, and even in the graphite of pencils we use for writing. The atomic number of carbon is 6 with an atomic mass of 12.01 g/mol. Positioned as a member of the 14th group, it ranks as the seventeenth most prevalent element on Earth.
Carbon occurs both freely and in combination with other elements. It exists in its elemental form as coal and graphite, while in combined states, it manifests as metal carbonates, hydrocarbons, and carbon dioxide gas. Combining with elements like hydrogen, oxygen, chlorine, and sulfur, carbon yields an impressive array of materials, ranging from tissues to medicines.
|
Catenation Property of Carbon
Organic Chemistry revolves entirely around carbon compounds, which are fundamental to all living organisms. Among the stable isotopes of carbon are 12C and 13C. Another isotope, 14C, is also present. Carbon-14 is utilized in radiocarbon dating due to its radioisotopic nature, featuring a half-life of 5770 years.
- One of the most amazing properties of carbon is its ability to make long carbon chains and rings. This property of carbon is known as catenation.
- Carbon has many special abilities out of all one unique ability is that carbon forms pπ-pπ bonds which are nothing but double or triple bonds with itself and with other electronegative atoms like oxygen and nitrogen.
- Just because of these two properties of carbon i.e catenation and multiple bond formation, it has the number of allotropic forms.
- Carbon atoms form tetravalent bonds , so that one carbon atom forms a bond with four carbon atoms and this structure can be repeated endlessly without disturbing the stability of the bonds.
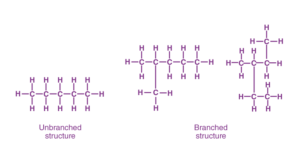
For more information you may visit : Catenation
Do you know what the allotrope of carbon means?
Allotropy refers to the existence of an element in multiple forms, each with distinct physical properties while maintaining similar chemical properties. These various forms are known as allotropes or allotropic forms. In the case of carbon, its allotropes are all based on carbon atoms but exhibit diverse physical characteristics, particularly in terms of hardness.
The primary crystalline allotropes of carbon include Diamond, Graphite, Graphene, Fullerene (C60), and Carbon nanotubes. Carbon demonstrates allotropy due to its existence in different forms such as diamond and graphite. However, it is now understood that all amorphous carbons contain microcrystals of graphite.
Despite having different crystal structures and physical properties, these carbon allotropes share the same chemical properties and exhibit similar chemical behaviors. Both diamond and graphite are represented by the symbol C, and they both produce carbon dioxide when strongly heated in the presence of oxygen.
The structures of carbon allotropes are diverse and include:
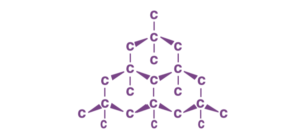
– Diamond: A three-dimensional network of carbon atoms arranged in a tetrahedral lattice, making it extremely hard.
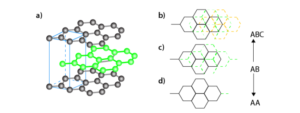
– Graphite: Consists of layers of carbon atoms arranged in hexagonal patterns, with weak forces between the layers, resulting in its characteristic lubricating and conducting properties.
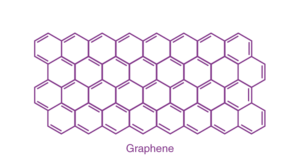
– Graphene: A single layer of graphite, arranged in a two-dimensional honeycomb lattice, known for its exceptional strength and electrical conductivity.
– Fullerene (C60): A molecule composed of 60 carbon atoms arranged in a spherical cage-like structure, exhibiting unique properties due to its hollow, cage-like shape.
– Carbon nanotubes: Cylindrical structures formed by rolling graphene sheets into tubes, known for their exceptional strength, thermal conductivity, and electrical properties.
Diamond:
Diamond is a well-known allotrope of carbon, featuring a three-dimensional network structure. It is renowned for possessing the highest hardness and thermal conductivity among carbon allotropes.
Graphite:
Graphite is another allotrope of carbon. It is a two dimensional structure and a good conductor of electricity.
Graphene:
It is a single layer allotrope of carbon having a two dimensional honeycomb structure. Graphene has very high electron mobility and, like graphite, is a good electrical conductor, due to the occurrence of a free pi (p) electron for each carbon atom.
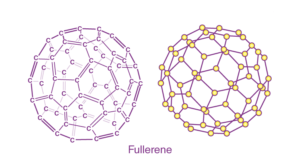
Fullerene (C6O):
A fullerene is an allotrope of carbon atoms connected by single and double bonds to form a closed or partially closed structure, with fused rings of five to seven atoms.
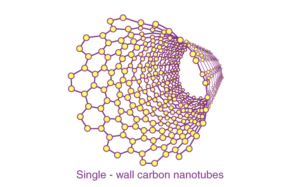
Single-wall carbon nanotubes:
Single-wall carbon nanotubes are one of the allotropes of carbon, intermediate between fullerene cages and flat graphene.
Some Carbon based compounds
Carbon -hydrogen compounds:
Hydrocarbons are the compound of carbon having a carbon hydrogen bond. Such as CH4, C2H6, C2H4, C6H6 etc.
Carbon-oxygen compounds:
There are several oxides of carbon compounds, including carbon dioxide (CO2), carbon monoxide (CO), and carbon trioxide (CO3), among others.
Carbon-sulfur compounds:
Some of the carbon-sulfide compounds include carbon disulfide (CS2) and carbonyl sulfide (OCS). Another example is carbon monosulfide (CS).
Carbon-nitrogen compounds
Examples of carbon-nitrogen compounds include cyanogen (C2N2), hydrogen cyanide (HCN), and cyanogen chloride (CNCl).
Carbon- halides compounds
The common carbon halides are carbon tetrafluoride (CF4), carbon tetrachloride (CCl4), carbon tetrabromide (CBr4), carbon tetraiodide (CI4) etc
Uses of carbon and its compounds
- Carbon is the basic building block of life.
- Hydrocarbons are the compounds of carbon and hydrogen are the major source of fuel.
- Carbon based compounds like ethylene, polypropene, polystyrene etc. are widely used in polymerisation process.
- They are used in the synthesis of many dyes and drugs.
- Diamond is a allotrope of carbon used for cutting marble, granite and glass, it is also used for jewellery.
- Graphite is used to make electrodes for electrolytic cells, for making pencils, lubricates for machines.
- Glucose is made of carbon , hydrogen and oxygen used is the main source of fuel for our cells

